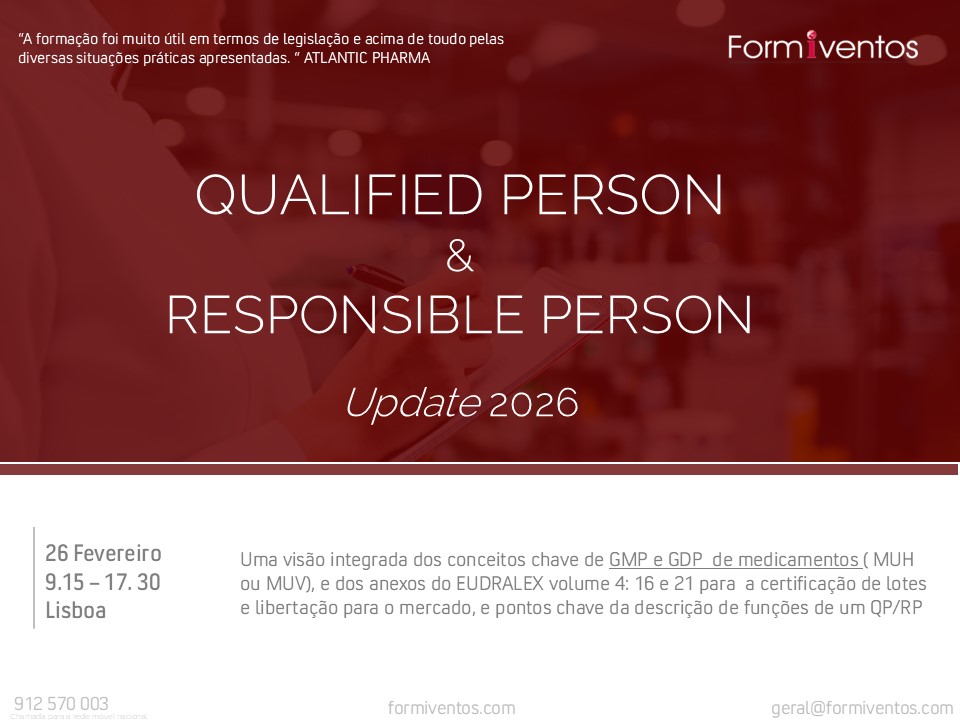
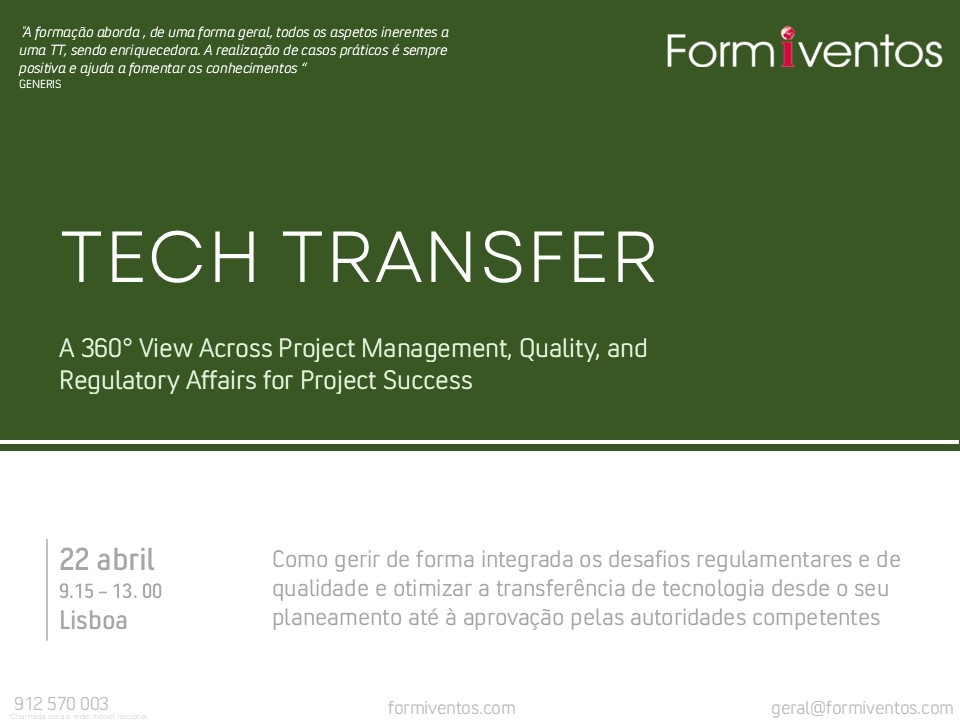
Sempre na vanguarda da melhor formação técnica dos profissionais da área Pharma , MedTech e Suplementos Alimentares

ÚLTIMAS NOTICIAS
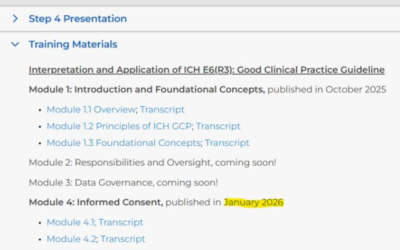
ICH E6(R3) update – New training materials available (Jan 2026)
The ICH Expert Working Group (EWG) has released additional training materials to support the implementation of ICH E6(R3), with a specific focus on Module 4: Informed Consent. Two transcripts are now available: 🔹 Module 4.1 (5 pages) Addresses key questions...
2025 EMA’s key recommendations in human medicines.
EMA have just published the overview of the 2025 EMA’s key recommendations in human medicines. It includes figures on the authorisation of medicines and a selection of new treatments that represent significant progress in their therapeutic areas. ✔️ 104...
Guiding principles of good AI practice in medicine development
EMA and the FDA have jointly identified 10 principles for good artificial intelligence practice in the medicines lifecycle. The use of AI technologies across the medicines lifecycle has increased significantly in recent years and holds great promise as a tool to...
European Pharmacopoeia publishes new data quality framework
The European Pharmacopoeia (Ph. Eur.) has published a new general chapter on Quality of data (5.38) in Issue 12.3, following its adoption by the European Pharmacopoeia Commission at its 182nd session in June 2025. New general chapter on quality of data 5.38 supports...

Comprometidos com a qualidade e a inovação.